Megaenzyme Engineering
NRPS
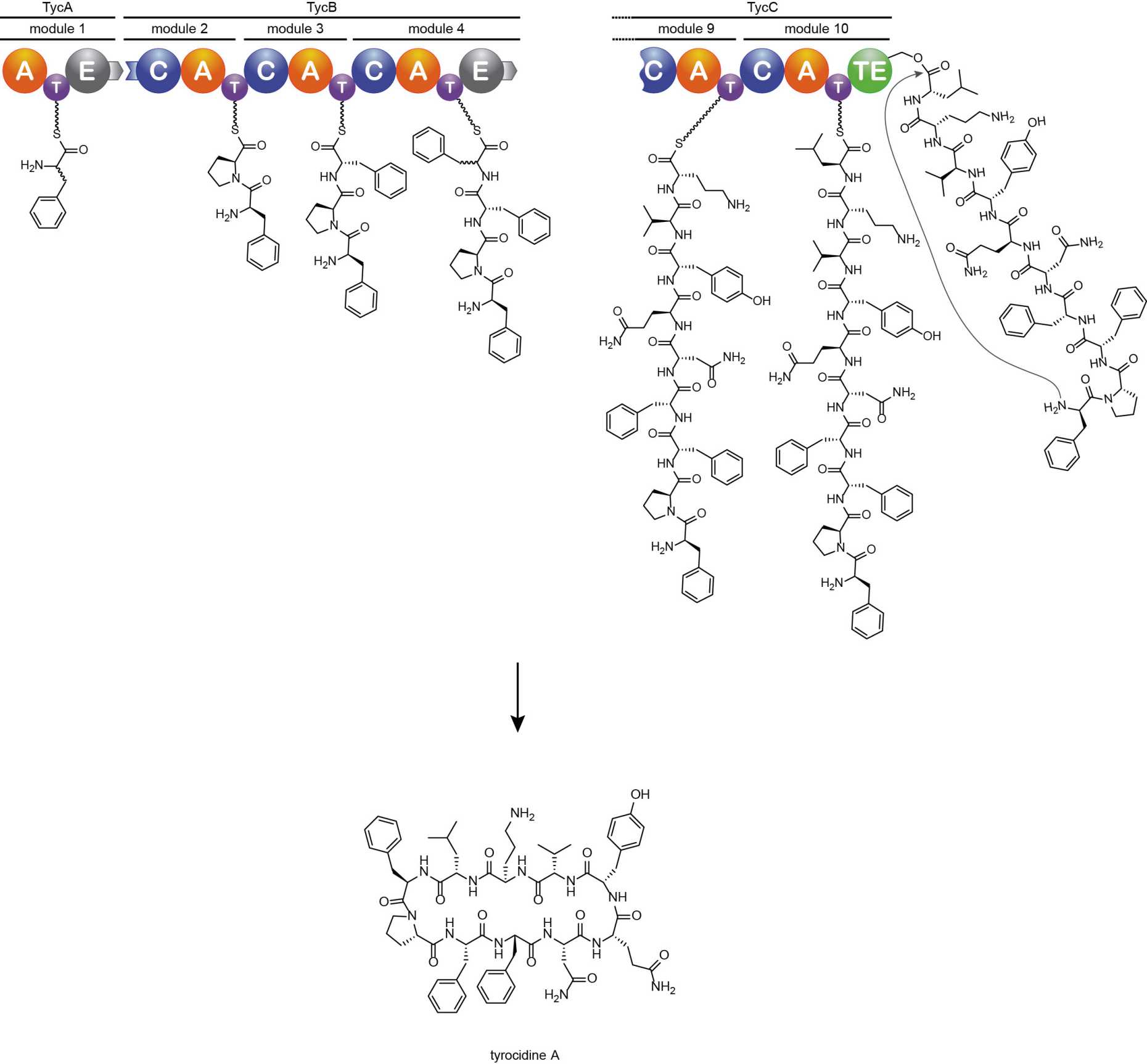
Nonribosomal peptide synthetases (NRPS) are mega-enzyme complexes that produce a wide range of biologically active natural compounds constituting an important source of useful therapeutics. They consist of multiple modules, which act in assembly-line fashion to incorporate the amino acid building blocks into the growing peptide chain. Each module contains several core domains that catalyze the individual reaction steps needed for amino acid activation, loading onto the enzyme, and peptide bond formation. The peptidyl product can be further diversified by inclusion of additional domains, for example for epimerization, cyclization, or oxidation/reduction. To enable repurposing NRPS domains for novel substrates, we are developing a toolbox of high-throughput methods to engineer individual domains. Efficient incorporation of “clickable” and β-amino acids into nonribosomal peptides illustrates the potential of such approaches for studying and engineering NRPS assembly lines for the production of novel tailored peptides.
Selected Publications
Kries, H. Wachtel, R., Pabst, A., Wanner, B., Niquille, D., Hilvert, D., Reprogramming nonribosomal peptide synthetases for “clickable” amino acids. Angew. Chem. Int. Ed., 53, 10105-10108 (2014).
Niquille, D. L., Hansen, D. A., Mori, T., Fercher, D., Kries, H., Hilvert, D. Nonribosomal biosynthesis of backbone-modified peptides. Nat. Chem., 10, 282 (2018).
Kries, H., Niquille, D. L., Hilvert, D. A subdomain swap strategy for reengineering nonribosomal peptides. Chem. Biol., 22, 640-648 (2015).
