Microfluidics
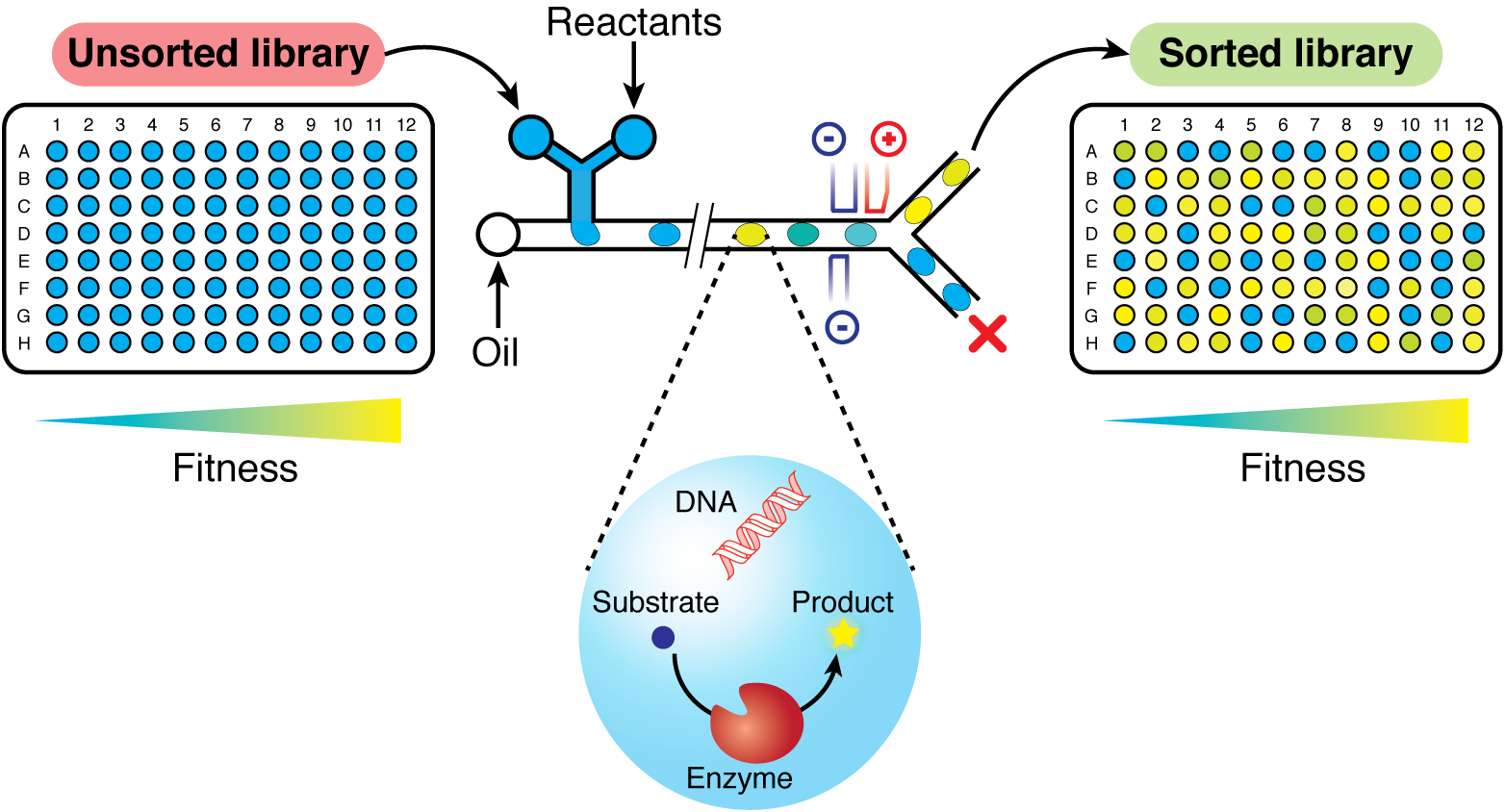
Analogous to a well on a microtiter plate, individual droplets in water-in-oil emulsions can be used as separate reaction compartments. Because droplets act as a diffusion barrier, compartmentalizing single cells producing an enzyme of interest together with a fluorogenic substrate and lysis agents physically links genotype and phenotype. Monodisperse droplets can be created and incubated for a defined length of time on microfluidic chips. Once the enzymatic reaction has taken place, droplets can be sorted according to their fluorescence signal. In this way, active variants can be isolated from libraries comprising several million inactive variants. We have adapted this technique to improve computationally designed retro-aldolases. Most notably, the increased screening power of FADS rescued the stalled evolution of retro-aldolase RA95.5-8 to give an enzyme variant that accelerated its target reaction >109 fold. Additionally, comparative evolutionary trajectories starting from the wild-type enzyme (RA95.0) showed that the increased throughput of FADS directly translates into larger improvements compared to microtiter plate based assays [2]. Currently, we are employing this system to parallelize the evolution of computationally designed aldolases from a vast array of different protein scaffolds to shed light on the relationship between protein fold and evolvability. Extension of microfluidics technology to different enzyme classes and its application to the rapid alteration of their substrate scope are currently being pursued.
Selected Publications
Obexer, R., A. Godina, X. Garrabou, P. R. Mittl, D. Baker, A. D. Griffiths and D. Hilvert. Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase. Nat. Chem. 9(1): 50-56 (2017).
Obexer, R., M. Pott, C. Zeymer, A. D. Griffiths and D. Hilvert. Efficient laboratory evolution of computationally designed enzymes with low starting activities using fluorescence-activated droplet sorting. Protein. Eng. Des. Sel. 29(9): 355-366 (2016).
